
News & Events
2020-03-03: Cyclica and the Institute of Materia Medica of the Chinese Academy of Medical Sciences carry out international cooperation to jointly promote the drug screening process of COVID-19
At present, the epidemic of novel coronavirus is sweeping all over the world, and more than 90 countries and regions are suffering from COVID-19. This rapid and large-scale spread makes more countries realize the urgency of international cooperation and response, and the situation of global war “epidemic” is taking shape. Recently, the Canadian AI medical company Cyclica and the Institute of Medicine of the Chinese Academy of Medical Sciences officially signed a strategic agreement on the cooperative research of antiviral drugs. The two sides have reached strategic cooperation intentions in COVID-19 drug screening, multi target drug design and other aspects. Through international cooperation, they will find drugs for 2019 novel coronavirus and promote the drug screening process of COVID-19.

The Institute of Pharmacy of the Chinese Academy of Medical Sciences is one of the key national drug research institutions in China. It has a complete drug research and development system and innovation strength, develops new drugs for the major needs of the clinical front-line, and has created many firsts in the field of drug research in China. In more than 60 years since its establishment in 1958, the Institute has developed and marketed more than 100 new drugs, obtained 130 new drug certificates, and won more than 230 awards for scientific and technological achievements as the first completed unit. It is a pioneer and pioneer of new drug research and development in China.
Cyclica is a biotechnology company headquartered in Toronto, Canada, and a typical high-tech enterprise under China Canada cross-border ecological cooperation. The company has gathered a world-class team with profound industry experience, and is committed to using biophysics and artificial intelligence (AI) to help the pharmaceutical industry carry out faster, safer and lower cost drug research and development. Cyclica has developed a series of comprehensive drug discovery platforms for pharmaceutical scientists – Ligand Express and Ligand Design, to change the current pharmaceutical industry plagued by high failure rates and high costs.
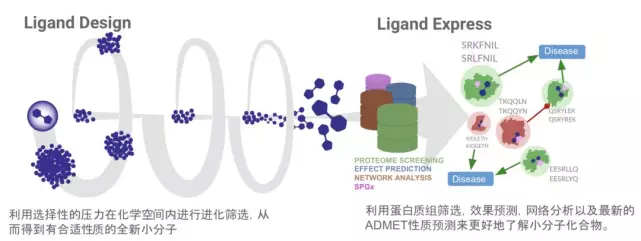
The picture shows the drug screening and design workflow of Cyclica Ligand Design and Ligand Express. Ligand Design and Ligand Express can be designed together to design the latest lead like molecules, so as to minimize unnecessary miss target effects, and comprehensively understand the molecular activity through integrated system biology and structural drug genomics. Cyclica’s differentiation platform provides new opportunities for drug discovery, including multi target drug design, lead compound optimization, ADMET attribute prediction, target deconvolution and drug reuse for various indications.
At present, Cyclica has cooperated with well-known research institutions and pharmaceutical enterprises in North America, Asia Pacific, Europe, South America and other regions. The publicly publicized partners include WuXi AppTec, Yuhan Pharmaceutical of South Korea, Eurofarma of Brazil, Bayer, Merck of Germany, etc., and have been highly recognized by the partners.
At present, there is no drug or vaccine officially approved by all countries for the treatment of novel coronavirus. Although the new drug therapy developed through innovative methods may have potential therapeutic effects on novel coronavirus, the development of new drugs will occupy a lot of resources and require years of preclinical and clinical testing. Therefore, “new use of old drugs” may be a more effective strategy at present to adapt to the urgency of urgent treatment of novel coronavirus pneumonia. The most typical example is remdeivir of Gilead Science, a nucleoside analog originally used to treat Ebola. For another example, Haizheng Pharmaceutical in China has been approved to test an influenza drug called favilavir to treat coronavirus. Artificial intelligence (AI) methods for drug reuse can further accelerate the recommendation of potential therapeutic solutions.
Cyclica used its proprietary in-depth learning engine MatchMaker to screen at least 6700 drugs and candidate drugs approved by FDA, which have at least Phase I clinical data. In addition to the protein structure data, MatchMaker has also been trained in millions of known human drug target interactions (DTI), resulting in a first-class database called PolypharmDB. This database is a unique collection for evaluating clinical molecules and their multi pharmacological characteristics. It can be quickly searched to identify potential candidate drugs to interact with selected protein targets.
Different from the traditional one-to-one calculation and modeling of drugs and targets, MatchMaker can quickly scan and sort all protein targets with structural descriptions, focusing on drugs, and match and screen a drug with all proteins in the proteome in a short time. In addition, MatchMaker is universal, and can screen any protein included in MatchMaker database, not limited to those common targets with a large number of training data. This uniqueness is of great significance for conquering new targets and targets rarely studied.
Cyclica used the flexibility of MatchMaker and PolypharmDB databases, based on the existing coronavirus structure and recent genome research, to model two 2019 COVID-19 proteins (3C protease – 3Cprot, S glycoprotein – spike glycoprotein) that have definite therapeutic relevance. By searching the expanded PolypharmDB database, it screened out the molecules that interact with the designated virus target. Using this method, Cyclica has obtained about 10 potential effective drugs that have been marketed or in clinical trials.
It is worth mentioning that the protein model targeted by Cyclica drug screening can be flexibly changed according to the discovery and establishment of key viral protein structure. That is, if the scientific community has new target suggestions, Cyclica can quickly screen new targets again.
In order to further screen the candidate list drugs for anti novel coronavirus biological activity in vivo and in vitro, Cylica CEO Naheed Kurji contacted the Chinese investment institution Zhongguancun Dahe Capital at the first time, hoping to receive relevant research institutions from China as soon as possible. Therefore, this strategic cooperation with the Institute of Medicine of the Chinese Academy of Medical Sciences was carried out.
According to the cooperation agreement, the Institute of Medicine of the Chinese Academy of Medical Sciences will screen the molecules proposed by Cyclica for antiviral activity in vitro and in vivo, and then determine promising candidate drugs for subsequent research and development. In addition, the two sides will work together to design multi-target antiviral compounds to reduce drug resistance.
This international cooperation is a further extension of the existing good relationship between Cyclica and China. The relationship between Cyclica and China originates from the CCAA China Canada Angel Alliance. CCAA China Canada Angel Alliance is an angel investment institution registered in Ontario, Canada. It was established in 2014 by Zhongguancun Dahe Capital and some angel investors of Zhongguancun Angel Hundred Association. It is committed to building a cross-border ecosystem between China and Canada, and promoting international exchanges and cooperation between Chinese and Canadian entrepreneurs through joint investment, entrepreneurial mentor services and various overseas channel resource docking. In 2015, Cyclica won the equity investment of CCAA and was selected into CAMP Sino Canada Joint Venture Camp for international virtual incubation; In May 2017, Cyclica performed well in the Zhongguancun U30 Overseas Special Competition, won the 2017 Zhongguancun U30 International Youth Innovation Award, and became a typical high-tech enterprise under China Canada cross-border ecological cooperation.
Naheed Kurji, CEO of Cyclica, said: “We have been paying close attention to the development of novel coronavirus in China and around the world. At the same time, we hope our AI platform can make contributions to this, in order to find a way conducive to rapid response to overcome the further outbreak of this virus. This cooperation with the leading scientists of the Institute of Medicine of the Chinese Academy of Medical Sciences is an opportunity we cannot miss.”
In the face of the coming novel coronavirus pneumonia, the global war “epidemic” situation is taking shape. It is becoming a global consensus to fight against COVID-19 and prevent a global pandemic by fully strengthening international cooperation in information communication, vaccine research and development, drug development and other fields.
As one of the countries affected by COVID-19, China actively carries out international cooperation and exchanges in response to COVID-19 with a responsible attitude and the principle of openness. Including timely release of data to the international community, open up the experience and practice in clinical medicine, actively promote the discussion with virologists and senior experts in various countries, and actively carry out international cooperation with global pharmaceutical manufacturers in terms of detection reagents, therapeutic drugs, vaccine research and development, etc. The signing of the Strategic Agreement on Cooperation in Antiviral Drug Research between Cyclica and the Institute of Medicine of the Chinese Academy of Medical Sciences is a typical example of international collaborative anti epidemic.


